How Many Valence Electrons Are in N3
16 valence electrons N3- 531 16 5 electrons of each nitrogen as there r 3 nitrogen here 5315 1 is the power on the ion Therefore there r total 16 valence electrons. There are 16 valence electrons for the Lewis structure for N3-.
Lewis Structure Of N3 With 6 Simple Steps To Draw
Atoms will tend to gain or lose electrons so that they acquire a noble gas electron configuration normally containing 8.

. Therefore a single elemental nitrogen has five valence electrons. There are a total of 16 valence electrons in the N 3- Lewis structure. That is in this case the valence of nitrogen ions is -3.
There are 16 valence electrons for the Lewis structure for N3-. This set describes an electron located on the third energy level in the 3d-subshell in the 3dz2 orbital and having spin down. The valence electrons are the electrons in the outermost shell in this case the 2nd shell.
The outer energy level is n 3 and there is one valence electron. How many core electron does n3- have. How many protons and electrons are in the N3 ion a protons 7 electrons 7 B protons 7 electrons 10 8 c protons 10 electrons 7 d protons 10 electrons 10.
Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. In the ion NO3 there is 1 atom of nitrogen and 3 atoms of oxygen. See the answer See the answer See the answer done loading.
Since each orbital can hold a maximum of two electrons the number of electrons that can share the two quantum number n 3 and ml 2 will be equal to 2 each having opposite spin. Step 1 of 3. Hence oxygen has 6 and nitrogen has 5 valence electrons in their outer shell.
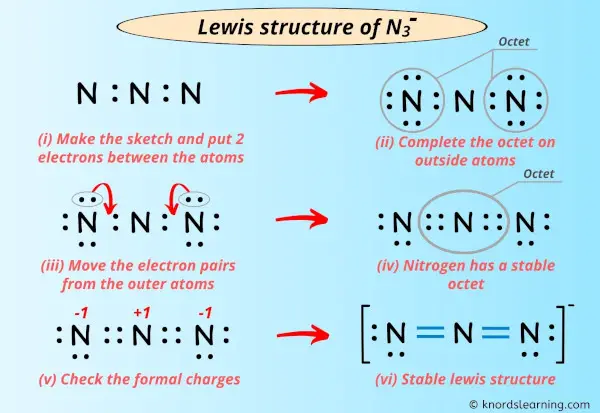
To clarify N3- has 8 valence electrons. How many total valence electrons are in N 3-. You should take formal charges into account with the Lewis structure for N3- to find the best structure for the molecule.
Get access to this video and our entire QA library. For the N3 Lewis structure calculate the total number of valence electrons for the N3 molecule. By signing up youll get thousands of step-by-step solutions to your homework questions.
Be sure to use the number of available valence electrons you found earlier. One ion of N 3 has valence electrons 3 5 1 1 6 42 g N 3 N A N 3 ion valence electrons in N A ions 1 6 N A where N A Avogadros number. Total electron pairs are determined by dividing the number total valence electrons by two.
The element sodium has the electron configuration 1s22s22p63s1. Answer 1 of 2. Nitrogen the element has 7 electrons.
The maximum number of electrons that can be contained in the n3 level is 18. 1 An atom with the electron configuration 1s22s22p2 possesses two valence electrons. Sp orbitals are oriented at 180 degrees to each other.
This is because Nitrogen has five electrons in its outermost shell and in order for it to gain a complete shell of eight it needs to gain three electrons. N 3l 2ml 2ms 1 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table.
Who are the experts. Two of the three statements are true. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit.
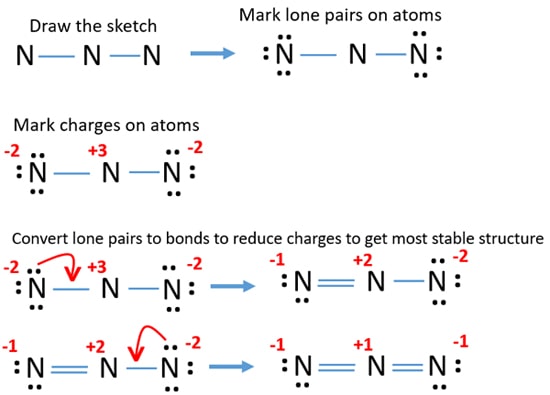
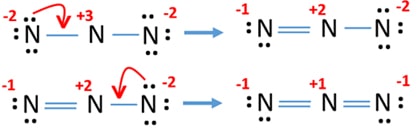
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. The attraction between this lone valence electron and the nucleus with 11 protons is shielded by the other 10 core electrons. Azide ion N3- lewis structure contains two NN bonds.
But for most of the transition and inner transition elements the valence electrons are the electrons present in the shells outside the noble gas core. Experts are tested by Chegg as specialists in their subject area. Valence electrons are those in the outermost shell of an atom.
Click hereto get an answer to your question The total number of valence electrons in 42 g of N3 - ion is NA is the Avogardos number. There are 16 valence electrons for the Lewis structure for N3-. For NO 2- there are 24 valence electrons so total pairs of electrons are 12.
2 The formula of the ionic compounds that contains Ba2 and N3- ions is Ba2N3. Ions are formed when atoms gain or lose valence electrons. How many protons and electrons are in N3-.
In sp hybridization the s orbital overlaps with only one p orbital. How many total valence electrons are in N3-. We review their content and use your feedback to keep.
Insulators 1 Nitrogen has 5 valence electrons 2 Sulphur has 6 valence electrons 3 Neon has 8 Valence electrons. There are a total of 16 valence electrons in the N 3- Lewis structure. This problem has been solved.
Total valence electrons 5 18 1 24. Total valence electrons pairs. There are three nitrogens and so 5 3 15 electrons for N X 3.
What is hybridisation of central atom. Draw the Lewis structure for N3- and draw a seperate lewis structure for BH3. 3 The polyatomic ions nitrate and azide are both tetraatomic entities.
This electron shell has enough energy to contain three sublevels. Simply add the 2 and the 6 together and you have 8 your answer. But two of those electrons are in the 1 s orbital and so are not considered valence.
The electron configuration shows that the nitrogen ion has acquired the electron configuration of neon. Answer and Explanation. Nitride ion is N with 3 charge.
The electron charge of Nitrogen N is negative three -3. Since the last shell of a nitrogen ion has eight electrons the valence electrons of nitrogen ion N 3- are eight. There are 16 valence electrons for the Lewis structure for N3- Thanks Think You Can Provide A Better Answer.
2 core electrons and 8 valence electrons are there in N3- ion. Also note that you should put the N3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.

The Number Of Dots Number Of Valence Electrons Ppt Download
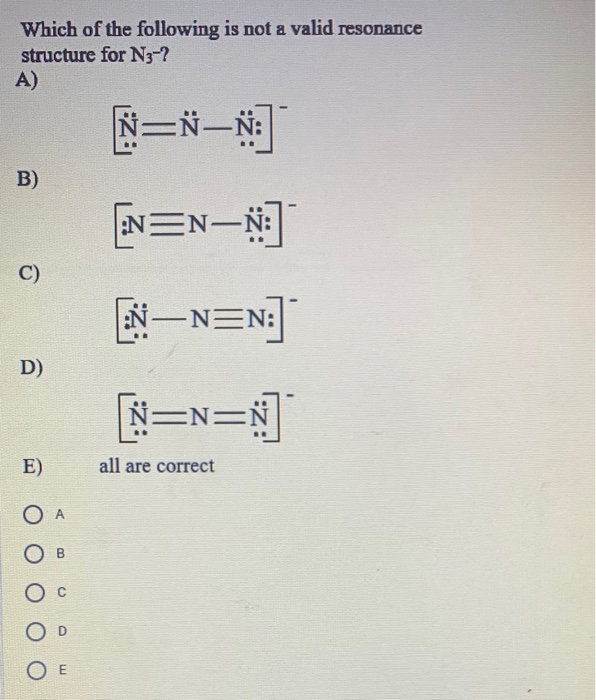
Solved Which Of The Following Is Not A Valid Resonance Chegg Com

Question Which Of The Lewis Structures Shown Below For Azide Ion N3 Has A Nitrogen Atom With A Formal Charge Of 2 A Structure I Only B Structure Ppt Download
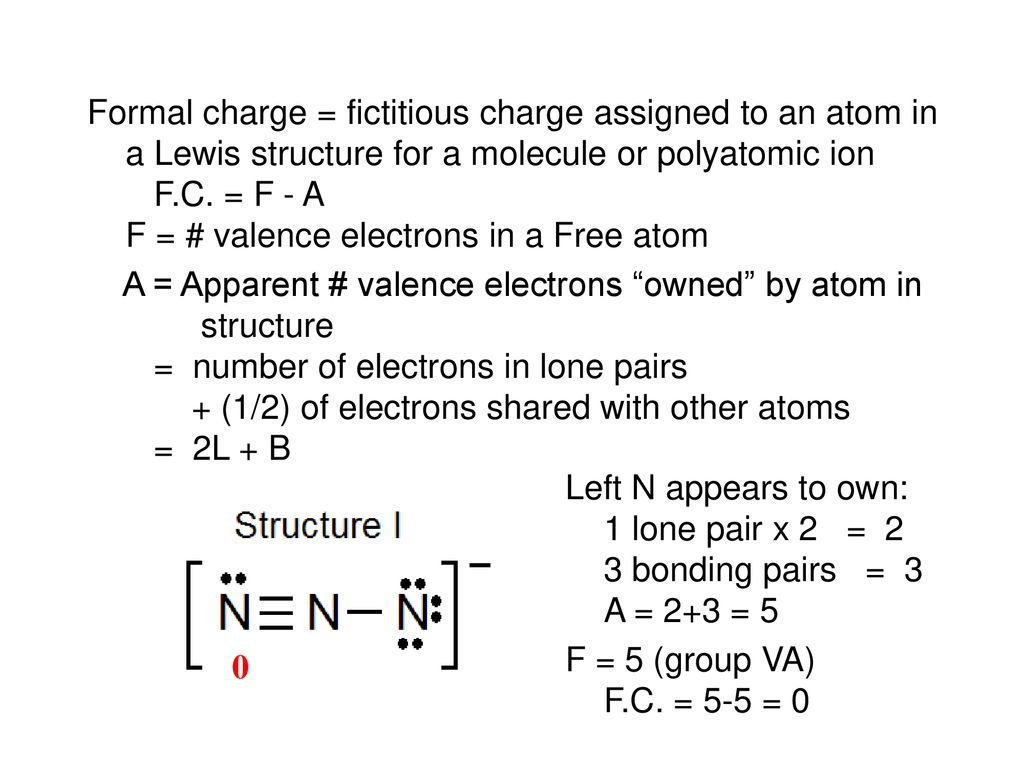
Question Which Of The Lewis Structures Shown Below For Azide Ion N3 Has A Nitrogen Atom With A Formal Charge Of 2 A Structure I Only B Structure Ppt Download

N3 Azide Ion Molecular Geometry Bond Angles Electron Geometry Youtube
Lewis Structure Of N3 With 6 Simple Steps To Draw
How Many Electrons Are Present In The Azide Ion Quora

The Total Number Of Valence Electrons In 4 2 G Of N3 Ion Is Na Is The Avogadro S Number Brainly In

How To Find Protons Electrons For The Nitride Ion N 3 Youtube

How To Draw The Lewis Dot Structure For N 3 Nitride Ion Youtube

How Many Resonance Structures Does N3 Have A 2 B 3 C 4 D No Resonance Study Com
N 3 Electron Configuration Nitride Ion Youtube

N3 Lewis Structure How To Draw The Lewis Structure For N3 Youtube

N3 Lewis Structure Azide Ion Youtube
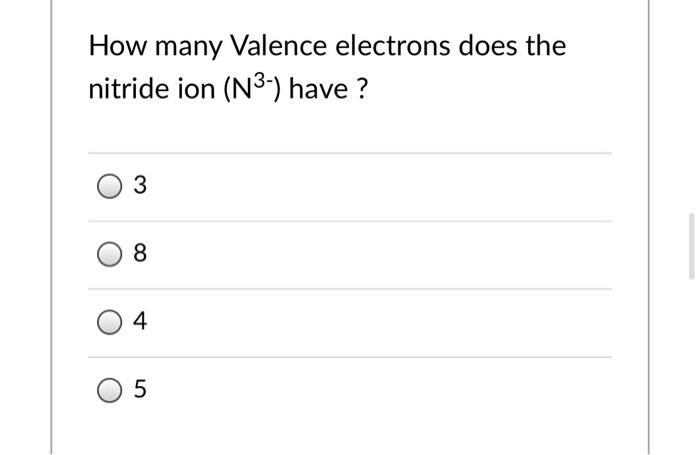
Solved How Many Valence Electrons Does The Nitride Ion N3 Chegg Com
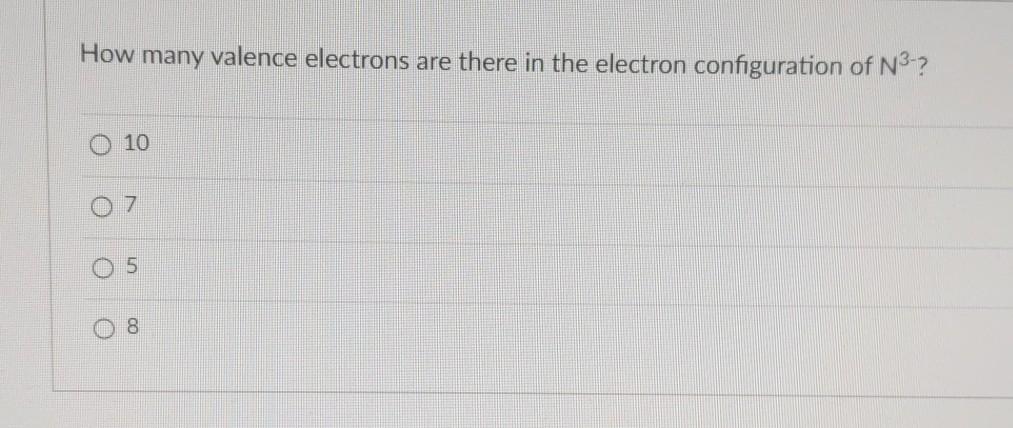
Solved How Many Valence Electrons Are There In The Electron Chegg Com
Comments
Post a Comment